BS EN 14683:Standard Requirements For Medical Face Mask

BS EN 14683-This European Standard specifies construction, design, performance requirements and test methods for medical face masks intended to limit the transmission of infective agents from staff to patients during surgicalprocedures and other medical settings with similar requirements. A medical face mask with an appropriate microbial barrier can also be effective in reducing the emission of infective agents from the nose and mouth of an asymptomatic carrier or a patient with clinical symptoms.
General requirements:
Materials and construction:The medical face mask is a medical device, generally composed of a filter layer that is placed, bonded or
moulded between layers of fabric. The medical face mask shall not disintegrate, split or tear during intended use.
Design:The medical face mask shall have a means by which it can be fitted closely over the nose, mouth and chin of the wearer and which ensures that the mask fits closely at the sides.
Performance requirements:
General:All tests shall be carried out on finished products or samples cut from finished products, if applicable in their sterile state.
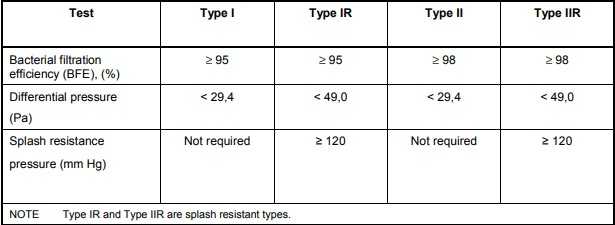
Bacterial filtration efficiency (BFE):When tested in accordance with Annex B, the bacterial filtration efficiency (BFE) of the medical face mask shall conform to the minimum value given for the relevant type in figure 1.
Breathability:When tested in accordance with Annex C, the differential pressure of the medical face mask shall conform to the value given for the relevant type in figure 1.
Splash resistance:When tested in accordance with ISO 22609 the resistance of the medical face mask to penetration of splashes of liquid shall conform to the minimum value given for Type IIR in figure 1.
Microbial cleanliness (Bioburden):When tested according to EN ISO 11737-1 the bioburden of the medical mask shall be ≤ 30 cfu/g tested
Biocompatibility:According to the definition and classification in EN ISO 10993-1, a medical face mask is a surface device with limited contact. The manufacturer shall complete the evaluation of the medical face mask according to EN ISO 10993-1 and determine the applicable toxicology testing regime. The results of testing should be documented according to the applicable parts of the EN ISO 10993 series.
2020-06-22 17:30
