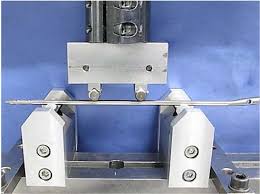
Intramedullary Nail Four-Point Bending Fatigue Tester
Standard:
ASTM F1264: Standard Specification and Test Methods for Intramedullary Fixation Devices.2 This is a primary standard specifically detailing static and fatigue testing methods for intramedullary nails, including four-point bending fatigue testing (often found in Annexes)
Relevant sections of ISO standards pertaining to the mechanical testing and fatigue life evaluation of surgical implants.4 These standards ensure that testing is conducted under controlled, reproducible conditions to assess the implant's ability to withstand cyclic loading without failure for a specified number of cycles.
Main applications:
Evaluating the fatigue life of intramedullary nails: Through long-term cyclic loading, measure the fatigue life of intramedullary nails under four-point bending conditions, that is, how many cycles of loading they can withstand without fracture or failure. This is a critical performance indicator for predicting implant longevity in vivo.
Studying the fatigue behavior of intramedullary nails: Observe and analyze phenomena such as the deformation and crack propagation of intramedullary nails during the fatigue process to understand their fatigue behavior and failure mechanism. This helps in identifying potential failure modes and optimizing nail design and material selection.
Quality control: During the production process, conduct four-point bending fatigue tests on intramedullary nails to ensure they meet quality standards and reduce the defective rate. Consistent fatigue performance is essential for ensuring the reliability of manufactured implants.
Material research and development: Provide an experimental platform for the research and development of orthopedic implant materials, supporting the development and application of new materials with improved fatigue resistance and mechanical properties suitable for intramedullary applications.
Product Advantages:
The Intramedullary Nail Four-Point Bending Fatigue Tester offers significant advantages for assessing the long-term performance of intramedullary nails:
Simulates Physiological Cyclic Loading: Replicates the dynamic bending stresses experienced by intramedullary nails in the body during activities, providing highly relevant fatigue data.
Determines Fatigue Life: Provides quantitative data on the number of cycles to failure, a critical parameter for implant longevity and safety.
Identifies Failure Mechanisms: Allows for analysis of how and where fatigue cracks initiate and propagate, informing design improvements.
Essential for Regulatory Compliance: Enables testing according to standards required by regulatory bodies (like FDA) for the approval of orthopedic implants.
Objective Durability Assessment: Offers a standardized and objective method for evaluating and comparing the fatigue performance of different intramedullary nail designs, materials, and manufacturing processes.
Accelerated Testing: Conducts fatigue tests over a shorter laboratory timeframe compared to the actual time an implant is in the body, while still providing valuable life expectancy data.
Product Features:
A typical Intramedullary Nail Four-Point Bending Fatigue Tester is based on a dynamic testing system and includes features tailored for implant fatigue evaluation:
A dynamic testing frame (e.g., servo-hydraulic or electrodynamic) capable of applying precisely controlled cyclic loads (force or displacement).
A four-point bending fixture specifically designed to accommodate intramedullary nails of various sizes and geometries.
Adjustable spans for the inner loading anvils and outer support anvils to meet standard requirements and accommodate different nail lengths.
A dynamic load cell for accurate measurement of the applied cyclic force.
A displacement sensor or extensometer for measuring the dynamic deflection of the nail during testing.
Advanced control system capable of applying various cyclic waveforms (e.g., sinusoidal, haversine) at specified frequencies and load/displacement amplitudes.
A high-speed data acquisition system to capture load, displacement, and cycle count data throughout the fatigue test.
Sophisticated software for setting up complex fatigue test profiles, real-time monitoring of test progress, detecting sample failure, and analyzing fatigue data (e.g., generating S-N curves).
A cycle counter to record the total number of load cycles applied.5
Often includes or is compatible with an environmental chamber or bath to perform tests at physiological temperature (e.g., 37°C) and potentially in a simulated body fluid environment.
Safety features such as overload protection, emergency stops, and protective enclosures.
Technical Parameters:
Dynamic Load Capacity: (e.g., typically ranging from ±5 kN to ±25 kN or more, suitable for applying physiological loads)
Frequency Range: (e.g., up to 10 Hz, 20 Hz, or higher, depending on the standard and test requirements)
Load Control Accuracy: (e.g., ±1% of set load or better)
Displacement Control Accuracy: (e.g., ±1% of set displacement or better)
Adjustable Inner Span (Loading Anvils): (Range covering relevant nail lengths and standard requirements)
Adjustable Outer Span (Support Anvils): (Range covering relevant nail lengths and standard requirements)
Maximum Number of Cycles: (Designed for long-term testing, e.g., up to 5 million or 10 million cycles as per standards)
Actuator Stroke: (Sufficient for applying required deflections)
Sample Size Capacity: (Able to accommodate the dimensions of various intramedullary nail types and sizes)
Power Supply: (e.g., AC 220V or 380V, 50/60 Hz, potentially with a hydraulic power unit if servo-hydraulic)
Leave Message Get Price











